Reduction of metal surfaces using atmospheric plasma
Atmospheric plasma is excellent for cleaning and reducing a broad variety of metal surfaces. The effect can be purely aesthetic and conservatory, e. g. when brightening or protecting valuable art objects or jewelry. In other cases, it is important to guarantee that a contact surface is free of oxides in order to achieve better bonding, for instance in roll cladding.
Reduction of metals
Metallurgical processes usually call for high temperatures. On the other hand, the reduction of metals is known to be possible at relatively low temperatures if the hydrogen offered has previously been dissociated by a plasma process. Energy input lets molecular hydrogen (H2) dissociate into atomic hydrogen, with required energy being ca. 435kJ/mol. At about 4000K, hydrogen is already more than 90% broken up into its atomic form. Atomic hydrogen is known to play a decisive role in reducing oxides (Bonhoeffer, 1924). The reduction process can be summed up in this molecular formula:
![]()
The reduction starts from the surface and is characterized by the diffusion of atomic hydrogen into the material’s lattice structure. The desorption kinetics of the water generated are equally important for effective depth penetration. The speed of conversion accelerates with rising temperatures and with a higher concentration of hydrogen atoms offered (Liebermann & Lichtenberg, 2005; Fridman, 2012). Therefore, surface reduction can excellently and effectively be achieved using atmospheric hydrogen plasma rich in excited atomic hydrogen.
Applied plasma system
For the technical-scale reduction of metal surfaces, the system most suited is one working with atmospheric plasma, which can be operated with different process gases or gas mixtures. It is of course an additional advantage if the performance, the gas mixture and the gas flow can be adjusted to the requirements of the processing steps.

Picture 2: Schematic representation of the roll cladding of a bimetallic strip. Reduction and cleaning of the surface with forming gas mixture (1), compression of the continuous strips (2) and thermal diffusion joining).
The practical requirements of the manufacturing process can be quite extensive. Sometimes, a precleaning process may be called for in addition to surface reduction, e. g. when organic residue needs to be removed via oxidation. The plasma system described here makes is possible to oxidize, clean and reduce a surface in a series of sequential steps. In order to prevent post-oxidation, the final process step can be carried out in a protective gas atmosphere.
Forming gas is a collective name for nitrogen (N2) and hydrogen (H2) gas mixtures with a mildly reductive effect. The term is deduced from an early application of such gas mixtures wherein a protective gas atmosphere was created for the in-mold annealing of spiral coils made of tungsten wire. Today, forming gas is used as a protective gas when metals are hot worked in processes such as soldering, welding (for root protection), rolling, pressing, and annealing. The hydrogen content prevents undesired oxidation. Forming gases are non-toxic, but can be flammable at higher concentrations. Therefore, a hydrogen content of more than 10% requires either flaring or dilution at the outlet nozzle where the forming gas exits.
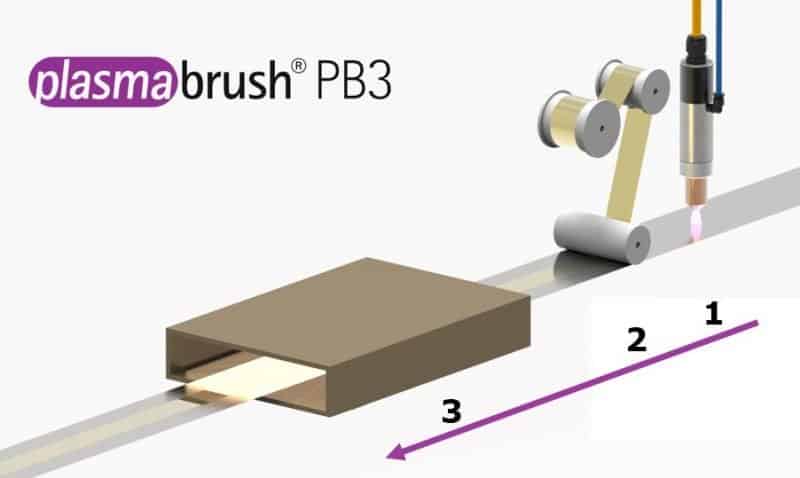
Picture 2: Schematic representation of the roll cladding of a bimetallic strip. Reduction and cleaning of the surface with forming gas mixture (1), compression of the continuous strips (2) and thermal diffusion joining).
Results exemplified by the process of plating
In metalworking, plating means the process of coating one or both sides of a base metal with one single or several layers of another metal. The aim is to create an extremely adhesive fusion (“plaqué“) which is made virtually inseparable through the use of pressure and/or subsequent heat treatment (e. g. diffusion annealing). Examples of possible bondings are copper on steel and silver or gold on brass (“doublé“), as well as many engineering alloys. Roll cladding is also very common for combining aluminium alloys with pure aluminium and steel with stainless steel, copper, nickel or aluminium.
Clad sheets are therefore composites of one basic material plated with at least one layer of another material. Either only one side or both sides of the carrier material can be coated with claddings. The desired effects may lie in optimized tensile strength, increased electrical conductivity or improved corrosion resistance compared with simple sheet metal, just to name a few examples. The quality of the bonding is essentially determined by how well the surfaces of the material interfaces are precleaned.

Picture 3: highly oxidized and contaminated belt material, consisting of a CuAg engineering alloy. In the middle, the effect of reductive plasma treatment can be observed.
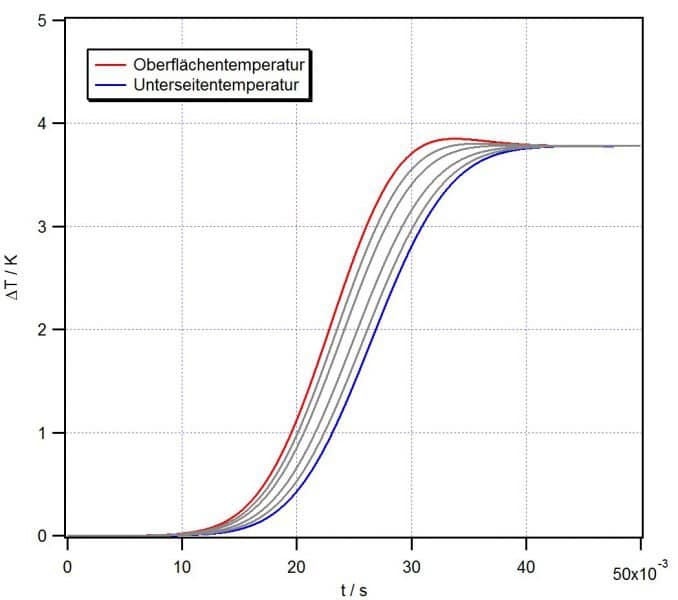
Transient surface temperature: the surface temperature of a copper belt 1mm thick and moving at a speed of 100mm/s rises only by about 4K if treated with plasma performing at 100W/cm2. This slight increase in temperature is due to the high thermal conductivity of the material.
For a continually running roll cladding plant, the integration of plasma substantially increased process stability and product quality, although the ingoing metal strips showed extremely fluctuating degrees of purity and oxidation.
Results exemplified by the treatment of precious objects
Atmospheric pressure plasma processes have a lot of potential when it comes to restoration and conservation. Both oxide and sulfite layers on silver can be reduced without ablating the silver. This technique is excellent for very fragile objects and combined objects like silver threads in textiles. Just as with silver, it is also possible to treat bronze, brass, nickel and many alloys this way in order to brighten them optically and prepare them with lasting effect.

Picture 4: Reduction of a silver special edition coin. Effect of the reductive plasma treatment (5% H2/95% N2) on the coin, which was half masked prior to treatment. The effect is achieved after only about 1 second of treatment (cf. Schmitt-Ott, 2009).
Summary
A large number of metals and alloys can excellently be cleaned, oxidized or efficiently reduced using atmospheric plasma processes. The reduction of metal surfaces with atmospheric plasma is suitable for single sensitive items such as cultural assets, coins, jewelry etc., or it can equally be operated in a continuous process. Role plating was used as an example to show the lasting effect of an atmospheric hydrogenous plasma jet.
Bibliography
Bonhoeffer, K. (1924). Zeitschrift der physikalischen Chemie, 113, S. 199.
Fridman, A. (2012). Plasma Chemistry. Cambridge: Cambridge University Press.
Liebermann, M. A., & Lichtenberg, A. (2005). Principles of Discharges and Materials Processing. New Jersey: Wiley.
Schmitt-Ott, K. (2009). Erhaltung von Kulturgütern – das Plasma in der Metallkonservierung – Möglichkeiten und Grenzen. Stuttgart: Staatliche Akademie der Bildenden Künste.




