Wetting
When we talk about wetting, we describe the behaviour of liquids on contact with the surface of solids. The corresponding property of the surface of the solid is the so-called wettability. How strongly a liquid wets a surface depends on various factors such as the type of liquid, material or surface or the nature of the surface.
Surface tension
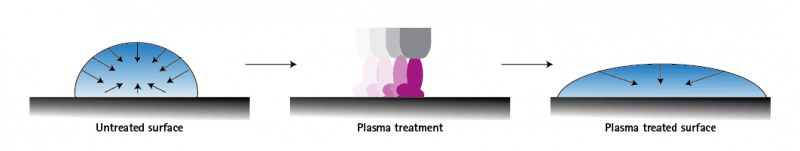
Wetting is directly related to surface tension. If the cohesion force within a water droplet is less than the adhesion force to the surface of the solid, the droplet spreads over the surface of the solid and the solid is completely wetted. If, on the other hand, the cohesive force is greater than the adhesive force, the water droplet takes on a spherical shape and the surface is hardly wetted.
Wettability
Many surfaces show insufficient wettability even in a clean state, which is intensified by impurities. As a result, the liquids roll off adhesives, lacquers and paints. This is because the surface tension is very low and insufficient for further processing. If unprocessed material is processed, the result is often that paint and varnish do not adhere properly and quickly come loose again or that stuck parts fall apart. This can be prevented with plasma activation. Plasma activation of a surface increases the surface energy of the surface and creates deposition points for the applied liquid. This allows it to adhere very well. Plasma activation modifies the surface and builds up surface energy, resulting in a improvement of significantly better wettability of the surface.
Learn about our products >>



