Polar interactions
The surface energy/tension of a substance is due to different interactions. It is composed of a disperse and a polar component. Polar interactions are strong and long-range interactions between molecules based on electrostatic attraction. In many molecules, the charge is not evenly distributed, but is present in the form of partial charges. Unequal charges attract each other, resulting in strong interactions between the charges.
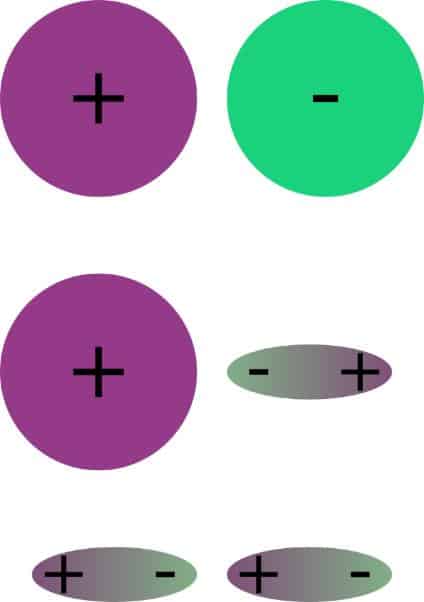
In polar interactions, a distinction is made between different contributions, which are of different strengths and have different ranges. The strongest interactions are between ions. With a distance dependence of 1/r, they also have a very high range. Then follow the interactions between a permanent dipole and ions with a distance dependence of 1/r². Much weaker are the dipole-dipole interactions. Here the distance dependence depends on whether the dipoles can rotate freely or not. A special case of the dipole-dipole interactions are the hydrogen bonds. These are very purposeful interactions that act between hydrogen atoms and negative partial charges. These strong interactions are the reason for the high surface tension of water. If there are no permanent charges, they can be induced. These disperse forces are much weaker than the polar ones, but they play an important role, e.g. in plastics.
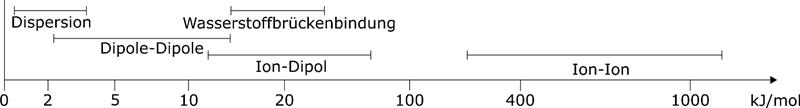
Strong interaction forces are especially important when stable compounds are involved. While disperse interactions are also possible without permanent dipoles, charges are required for polar interactions. In particular, so-called low-energy surfaces with a low surface energy and a low polar fraction often cannot be further processed without pretreatment. One possibility for pretreatment is plasma treatment, in which reactive species remove the finest impurities from the surface and additionally functionalize the surface by the addition of polar groups. The polar groups contribute to the polar interactions with their dipole moment, which is also reflected in the polar fraction of the surface energy. In many applications, it has been shown that only a sufficiently large polar fraction enables surfaces to be wetted with many coatings, adhesives and other substances.
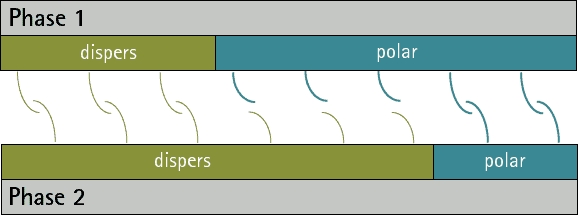
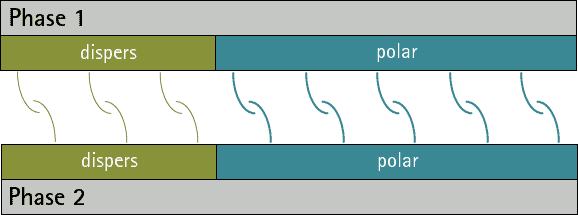
There can be no substances whose surface tension/energy is only polar, since disperse interactions occur in all atoms and molecules. However, there are substances whose surface energy/voltage is purely disperse, since they have no polar groups.
By comparing the disperse and polar fractions of two phases, predictions can be made about their adhesion to each other. The more the disperse and polar fractions match, the more interaction possibilities there are between the phases and the stronger adhesion can then be expected (see figures 1 and 2). A low interfacial tension/energy is evident when there is a high interaction potential between two phases.
Learn about our products >>



