Ozone
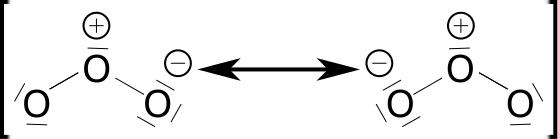
Ozone (O3) is a molecule made up of three oxygen atoms and is a strong oxidant. Ozone is not stable and decomposes into oxygen within a few minutes to a few days, depending on conditions. It has a mesomeric boundary structure with a partially positive charged central atom and is therefore, unlike most gases, polar.
Ozon for disinfection
Due to its oxidative properties, it irritates the mucous membranes, but can also be used in many applications. As a strong oxidant, O3 inactivates many germs, which is why it is used as a disinfectant. Furthermore it is also used for water treatment, both for industrial water and swimming pool water.
Advantages
The advantage of ozone as a disinfectant is that it can be produced on the premises without the need to handle chemicals. In addition, it is broken down into harmless oxygen. A major problem is odours, which are largely based on volatile organic compounds (VOC). O3 oxidises these compounds to odourless carbon dioxide and water. Ozone is produced by discharges, such as plasma, or by UV-C radiation which leads to the photodissociation of oxygen.
Learn about our products >>



