Piezoeletric cold atmospheric plasma induces apoptosis and autophagy
in human hepatocellular carcinoma cells through blocking glycolysis and AKT/mTOR/HIF-1α pathway
Authors: Yanhong Wang, Xinyu Mang, Danni Li, Yiliang Chen, Zhenyu Cai, Fei Tan
Date: 1 November 2023
First published on: https://www.sciencedirect.com/science/article/pii/S0891584923005701?via%3Dihub
Abstract
Hepatocellular carcinoma (HCC) is the sixth most prevalent cancer and the fourth leading cause of cancer-related death worldwide. Advanced or metastatic HCC is currently managed using systemic drug therapy with unsatisfactory patient survival. Cold atmospheric plasma has emerged as a promising, physicochemical, and broadspectrum oncotherapy. In this preclinical study, we investigated the anti-neoplastic functions and mechanism of piezoelectric direct discharge technology-based CAP, Piezo-CAP, on HCC in vitro and in vivo. Various HCC cells lines, such as SMMC7721, HepG2 and LM3, were used as in vitro cancer model for the phenotypic and mechanistic studies.
Introduction
Cold atmospheric plasma (CAP) has been recently recognized as a physicochemical anti-neoplastic therapy. As the fourth state of matter, CAP is a partially ionized and non-thermal gas mixture generated at room temperature under ambient pressure. Plasma oncology, i.e., applying CAP for tumor treatment, along with successful applications of CAP in other medical and surgical specialties, have attracted tremendous interest. The foundation of plasma oncology is laid upon the internal vulnerability of redox disbalance, i.e., abnormally elevated cancer cellular reactive oxygen species (ROS), and external targeting by the predominant component of CAP, i.e., reactive oxygen and nitrogen species (RONS) [14]. In addition to these chemical factors, other physical constituents of CAP, such as thermal discharge, ultraviolet radiation, and electromagnetic waves, might also trigger cancer cell death, further complexing the role of CAP as a physicochemical anti-cancer treatment.
Piezo-CAP inhibits the proliferation of HCC cells
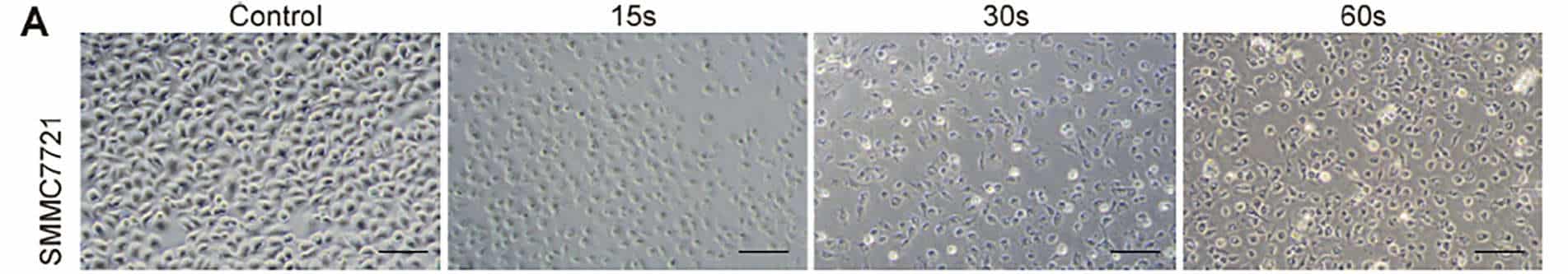
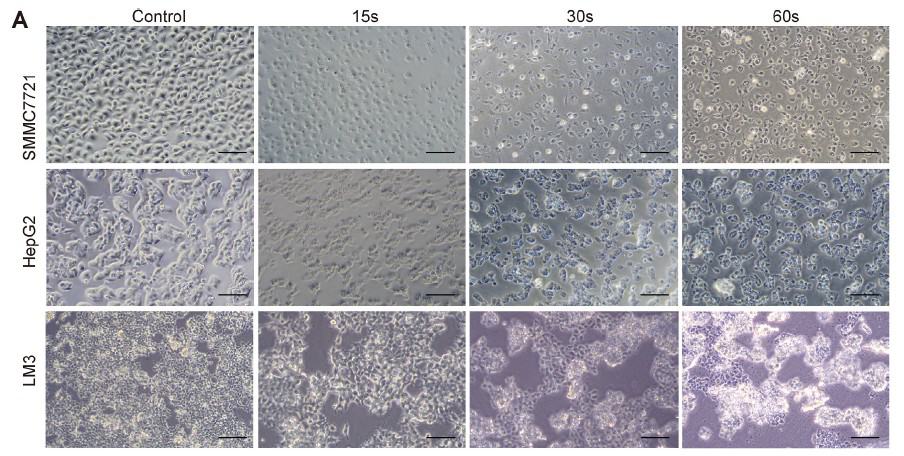
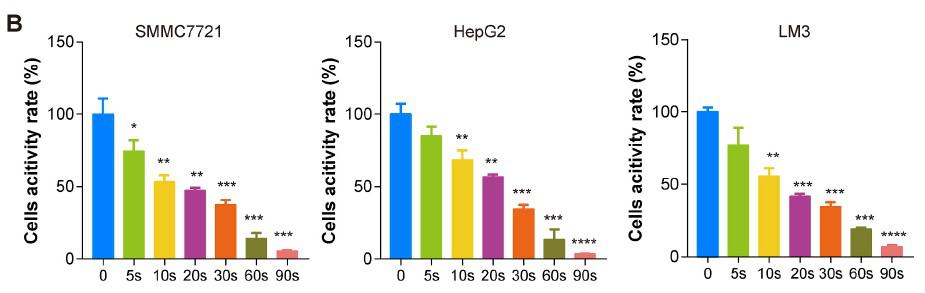
In order to examine the universal effect of Piezo-CAP on the proliferation of various types of HCC cells, exponentially growing SMMC7721, HepG2 and LM3 cells were treated with increasing duration of plasma (from 5 s to 90 s). There appeared to be obvious change in the morphology and cell density in the tested HCC cells under phase contrast microscopy (Fig. 2A). All the cells were significantly shrunk and less adherent to the cell culture dish after treatment by Piezo-CAP for 30s. When the treatment duration was doubled, the cells became spherical, shriveled and visually suspended in the cell culture medium, suggesting more prominent cell detachment. In addition, the effect of Piezo-CAP on cell proliferation was quantitatively confirmed using CCK-8 assay (Fig. 2B) and colony formation assay (Fig. 2C and D). Piezo-CAP significantly inhibited the proliferation of all HCC cells tested when compared with the control (P < 0.01). The IC50 values of SMMC7721, HepG2 and LM3 cells
Darüber hinaus wurde die Wirkung von Piezo-CAP auf die Zellproliferation mit Hilfe des CCK-8-Tests (Abb. 2B) und des Koloniebildungstests (Abb. 2C und D) quantitativ bestätigt. Piezo-CAP hemmte signifikant die Proliferation aller getesteten HCC-Zellen im Vergleich zur Kontrolle (P < 0,01). Die IC50-Werte von SMMC7721-, HepG2- und LM3-Zellen nach Piezo-CAP-Behandlung lagen bei 19,73, 22,81 bzw. 19,47.
Conclusion
In this preclinical study, we are the first to systematically demonstrate potent anti-cancer effect of piezoelectric cold atmospheric plasma on human hepatocellular carcinoma and the underlying molecular mechanism. Using various in vitro models (SMMC7721, HepG2 and LM3 HCC cells) and in vivo model (CDX mice), we first established that Piezo-CAP exerts anti-tumor functions through inhibiting various cancer hallmarks, such as cell proliferation, migration and invasion, EMT, and antioxidant defense. Further phenotypic and mechanistic studies revealed Piezo-CAP’s two-pronged anti-cancer strategy: inducing cancer cell death and disrupting cancer cell survival. The former is exemplified by plasma-induced multiform programmed cell death, e.g., apoptosis and autophagy, whereas the latter by plasma-attenuated survival mechanism, e.g., tumor glycolysis and oncogenic proliferation. Assisted by bioinformatic analysis, we discovered and verified numerous signaling pathways that are sensitive to Piezo-CAP treatment of HCC, such as oxidation-reduction pathway of GSH, E-cadherin/N-cadherin
switch of EMT, mitochondrial apoptotic pathway, glycolytic pathway, and PI3K/Akt/mTOR/HIF pathway. Most importantly, Piezo-CAP achieved aforementioned anti-HCC functions with exceptional power efficiency, compactness and versatility, and without additional gas requirement other than just ambient air. In conclusion, Piezo-CAP is a promising, adjunctive, physicochemical anti-HCC therapy. Based on the
transcriptomic, protein, cellular and animal data from this study, the ideal next stage would be preparation for human clinical trials.
You can read the full article Piezoelectric cold atmospheric plasma induces apoptosis and autophagy here.